What is matter?
Matter is anything that takes up space and has mass.
What is an element?
An element is the simplest substance known – it cannot be broken down by chemical means into another substance. Elements can be metals (e.g. gold, silver, iron, copper) or non-metals (e.g. hydrogen, oxygen, carbon, nitrogen). There are a total of 118 elements.
Have you seen a periodic table? The periodic table is just every one of the 118 elements arranged in a table.
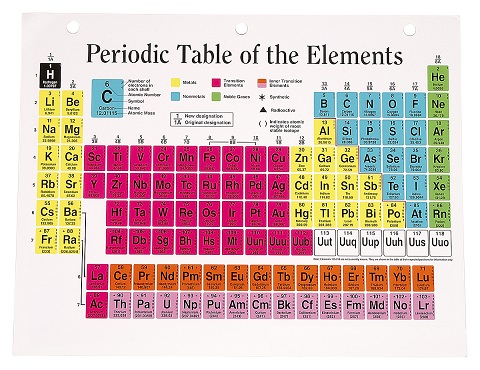
Chemical elements arranged in a periodic table
What are atoms?
Atoms are the basic building blocks of matter.
All matter is made up of atoms, be it solids, liquids or gases. All things around you, things you can and can’t see (the air you breathe), even you, are made up of atoms.
Atoms are tiny particles – there are millions, billions, trillions of atoms in the smallest speck of any matter around you. The atom is also the smallest particle in an element that still has the properties of the element. For example, the atoms of element aluminum cannot be broken down further. Each atom of it will have the properties of aluminum.

Atoms packed into matter
What are molecules?
A molecule is the smallest amount of a chemical substance which can exist by itself. Molecules are formed by the combination of two or more atoms. Atoms join together, or as we say in chemistry, they form bonds together, to make molecules.
Examples:
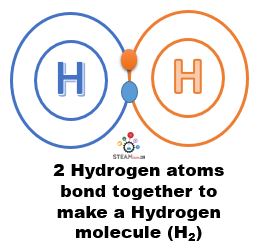
- An atom of hydrogen is not stable by itself. So, two atoms of hydrogen bond together to form a molecule of hydrogen, which can exist by itself. This molecule is termed H2 because it contains 2 atoms of hydrogen.
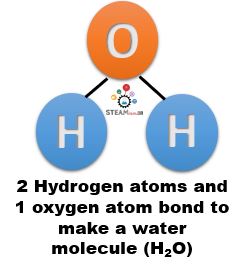
- 2 atoms of hydrogen bond with 1 atom of oxygen to form a molecule of H2O, which is water!
Fun activity to understand atoms and elements
Here’s a fun activity to do at home!

- Take a piece of aluminum foil and rip into small pieces. Aluminum is your element.
- Keep ripping until it cannot be ripped into any smaller pieces.
- Imagine you’ve ripped it into the smallest possible piece of aluminum. This is your atom (not really – this tiny piece has millions of atoms in reality). It has all the properties of aluminum.
- If you ripped this piece (atom) again, it would no longer be aluminum or have its properties.
- How To Write A Lab Report - April 8, 2023
- The States (Phases) of Matter - June 9, 2020
- What is Engineering? - June 2, 2020