How is matter classified?
All matter can be classified as:
- Elements
- Compounds
- Mixtures
Wait-a-second, why not classify matter as phases?
Some substances, like water can exist in more than one phase
- Solid-ice
- Liquid- water
- Gas – steam
Some substances can also easily change from 1 phase to another.
So, classifying matter by phase is not very useful to us scientists, is it?
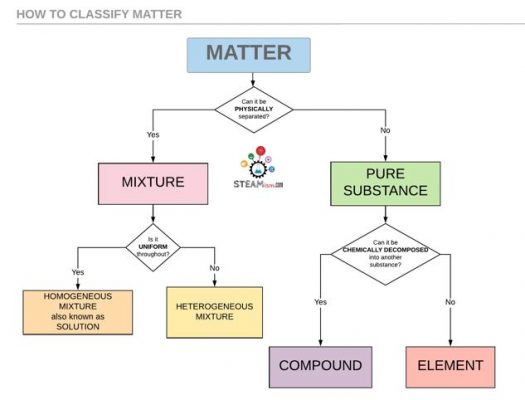
You can classify all matter using 3 questions
- Can it be physically separated?
- Can it be chemically separated?
- Is it uniform throughout?
Here’s a cool flowchart that shows how you can use these questions in an easy and logical way to classify matter.
What are Elements?
Elements are the simplest form of a pure substance. They are represented by chemical symbols, consisting of one or two letters.
- They cannot be broken into anything else by physical or chemical means.
- The smallest particle of an element that has all the properties of that element is an atom. All atoms of an element are alike.
- There are a total of 118 known elements, 94 of which occur naturally on Earth.
- These elements are organized in a logical manner on a table called The Periodic Table.
- These elements combine together to create millions of compounds.
Common Elements in the Earth’s crust
The 9 most common elements in the Earth’s crust are –
- Oxygen ~ 46%
- Silicon ~ 28%
- Aluminum ~ 8.2%
- Iron ~ 5.6%
- Calcium ~ 4.2%
- Sodium ~ 2.5%
- Magnesium ~ 2.4%
- Potassium ~ 2.0%
- Titanium ~ 0.61%.
What are compounds?
Compounds are also pure substances, but they are made from more than one element.
- They are usually the unions of two or more elements.
- They can be broken into simpler substances by chemical means.
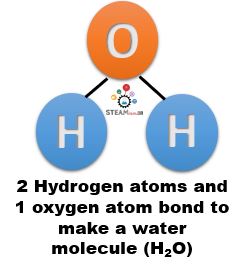
- Example – Water (H2O) is a compound. It can be broken down into simpler substances – hydrogen and oxygen.
What are mixtures?
If a substance can be physically separated, it is classified as a mixture.

- The substances in a mixture retain their individual properties.
- Is this mixture uniform throughout?
- No? Then it’s a heterogeneous mixture.
- Examples are – trail mix, sand and pebbles mixed together, oil in water.
- Suspensions are a type of heterogeneous mixture (fine sand in water)
- Yes? Then it’s a homogeneous mixture. It looks the same throughout. Particles are very small and look well-mixed together. These can be classified into –
- Examples are – corn oil, white vinegar, sugar solution (mixed well so all you see is a clear liquid).
- A colloid is a homogeneous solution with intermediate particle size. So if you shone a beam of light through it, the particles would be visible. Milk, fog, and jello are examples of colloids.
- No? Then it’s a heterogeneous mixture.
Latest posts by Divya Pochimcherla (see all)
- How To Write A Lab Report - April 8, 2023
- The States (Phases) of Matter - June 9, 2020
- What is Engineering? - June 2, 2020